Time:2014-06-03ClickTimes:
Water-solid interactions are of broad importance both in nature and
technology. The hexagonal bilayer model based on the Bernal-Fowler-Pauling ice
rules has been widely adopted to describe water structuring at interfaces.
However, the validity of the hexagonal bilayer model on interfacial water is
being challenged due to the subtle balance between the water-water and
water-substrate interactions. Recently, the research team led by Prof. Ying
Jiang of International Center of Quantum Materials (ICQM) of Peking University,
in collaboration with Prof. Enge Wang and Prof. Xinzheng Li from the same
center, discovered a highly defective tetramer-based ice structure on NaCl(001)
surface, which goes well beyond the simple hexagonal bilayer model predicted by
the ice rules. This work was published online in Nature Communications on May
30th, 2014 [Nat. Commun. DOI: 10.1038/ncomms5056].
During the past four years, Ying Jiang’s group has been working on the
development of ultrahigh-resolution scanning tunneling microscope (STM) for
single-molecule experiments. Recently, they made a breakthrough in achieving
submolecular-resolution imaging of individual water molecules adsorbed on a
Au-supported NaCl(001) film [Nat. Mater. 13, 184 (2014)]. Such a technique opens
up the possibility of determining the detailed topology of H-bonded networks at
water/solid interfaces with atomic precision.
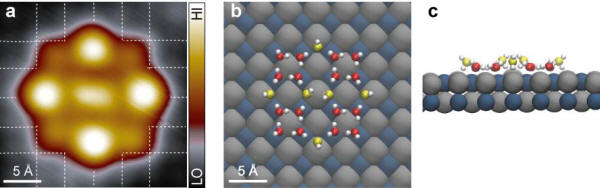
|
(a) High-resolution STM image of a 2D ice nanocluster consisting of
four tetramers and six bridging water
molecules. Square lattices of the NaCl(001) surface arising from Cl are
depicted by white grids. (b) and
(c) Top and side views, respectively, of the calculated adsorption
configurations of the ice nanocluster.
H, Cl and Na are denoted by white, grey and dark-cyan spheres,
respectively. For clarity, the O atoms of
water molecules in lower and upper layers are represented by red and
yellow spheres, respectively.
|
As an important application of this technique, Ying Jiang and his
collaborators stepped further to study the ice overlayer grown on the NaCl(001)
film and discovered an unconventional bilayer ice structure built from cyclic
water tetramers at 77 K. The water tetramers within the lower part of the
bilayer act as the basic building blocks, which are interconnected via a novel
bridging mechanism to form a regular array of Bjerrum D-type defects located in
the upper layer. Ab initio theoretical calculations based on density functional
theory rationalize the stabilization of such an unconventional bilayer ice and
reveal a striking proton-disordered ice structure. Notably, the formation of the
periodic Bjerrum defects with unusually high density is strongly against the
Bernal-Fowler-Pauling ice rules and may play a crucial role in catalyzing
heterogeneous chemical reactions on water-coated salt surfaces as well as in
influencing various phenomena such as heterogeneous ice nucleation, salt
dissolution and caking.
This work received supports from Ministry of Science and Technology of China,
National Natural Science Foundation of China, Ministry of Education of China,
and National Program for Support of Eminent Professionals.
Paper link:http://www.nature.com/ncomms/2014/140530/ncomms5056/full/ncomms5056.html
